FDA Approvals Mark Major Advances in Cancer Treatment: Pembrolizumab Breakthroughs
In January 2024, the FDA achieved a significant milestone by approving two pivotal drugs for cancer treatment. On January 12, pembrolizumab (Keytruda, Merck), in combination with chemoradiotherapy, received FDA approval for the treatment of FIGO 2014 Stage III-IVA cervical cancer. This was followed by the successful approval of erdafitinib (Balversa, Janssen Biotech) on January 19, a drug designated for use in cases of locally advanced or metastatic urothelial carcinoma.
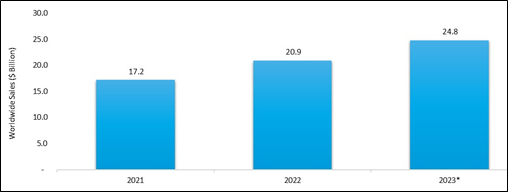
Keytruda (Merck & Co) Worldwide Sales, 2021-2023 ($ Billion)
Merck presently has numerous candidates undergoing regulatory review both in the United States and internationally, or they are in the advanced stages of clinical development. Keytruda (MK-3475), an anti-PD-1 therapy, has gained approval for treating various cancers and is currently undergoing clinical development for expanded indications. The approvals stem from an extensive clinical development initiative that encompasses over 1,650 clinical trials, with more than 1,200 trials investigating Keytruda in combination with other cancer treatments. These trials span over 30 different cancer types, including biliary, estrogen receptor-positive breast cancer, cervical, colorectal, cutaneous squamous cell, endometrial, esophageal, gastric, glioblastoma, head and neck, hepatocellular, Hodgkin lymphoma, non-Hodgkin lymphoma, non-small-cell lung, small-cell lung, melanoma, mesothelioma, ovarian, prostate, renal, triple-negative breast, and urothelial cancers. Many of these trials are currently in Phase 3 clinical development, and additional trials for other cancers are in the planning stages.
Currently, Keytruda is FDA-approved for the treatment of patients with previously treated advanced HCC. Keytruda also received a priority grant from the FDA for its potential use in combination with Padcev (enfortumab vedotin-ejfv) to treat specific patients diagnosed with locally advanced or metastatic urothelial cancer, who are not suitable candidates for cisplatin-containing chemotherapy.
Sales Data 2023
- Keytruda is projected to register 18.7% sales growth in 2023 compared to 2022.
- In the first nine months of 2023, Merck's Keytruda sales were $18 billion.
- In the first half of 2023, Keytruda sales were $12.07 billion, a 19.9% increase.
- In the third quarter of 2023, Keytruda sales were $6.3 billion.
FDA Approval Update 2023
- Keytruda is FDA-approved for treating recurrent locally advanced or metastatic Merkel cell carcinoma in both adult and pediatric patients.
- The FDA is also FDA-approved Keytruda for its potential use in treating adult and pediatric patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) solid tumors. This applies to cases determined by an FDA-approved test and where the tumors have progressed after prior treatment, with no satisfactory alternative treatment options available.
- In the European Union, Keytruda is approved for its potential as adjuvant treatment in patients with stage IB (?4 cm), II, or IIIA non-small cell lung cancer (NSCLC) following complete surgical resection.
- In Japan, Keytruda is also approved for treating patients with relapsed or refractory primary mediastinal large B-cell lymphoma (PMBLC).