Global Point-of-Care Molecular Diagnostics Market to Become $5.6 Billion by 2030
The point-of-care (POC) diagnostics are medical devices used to get an immediate result in the investigation (diagnosis & monitoring) of various diseases, such as cancer, diabetes, cardiac diseases, and others. However, Molecular diagnostics involves taking DNA or RNA, the unique genetic code found in our cells, and analyzing the sequences for red flags that can pinpoint the potential emergence of a specific disease.
The point-of-care (POC) molecular diagnostics market is reported to grow significantly in the forecasted period due to rising disease prevalence and the growing technology to improve disease diagnostics in the laboratory sector in healthcare.
The market is segmented by Application (influenza, mononucleosis, respiratory infectious disease, gastrointestinal infectious disease, sexually transmitted disease, and others such as prenatal testing and endocrinology), Technology (PCR, genetic sequencing, hybridization, microarray, and INAAT), End-User (Hospitals, Homecare, and Other End Users), and Geography (North America, Europe, Asia-Pacific, Middle-East and Africa, and Latin America).
COVID-19 significantly increased the global need for diagnoses. Rapid diagnostic test kits to identify the virus in clinical specimens are in high demand due to the exponential growth in COVID-19 infections. For instance, The first COVID-19 diagnostic test, Lucira COVID-19 All-In-One Test Kit for self-testing at home with quick results, received an emergency use permission (EUA) from the US Food and Drug Administration (FDA) in November 2020. It is a molecular test with a single-use goal of identifying the new coronavirus SARS-CoV-2 that produces COVID-19 due to the market's broad commercialization of POC COVID-19 diagnostics.
However, there are several limits to molecular diagnostic technology. To stop the spread of the disease, a precise diagnosis is essential. This research shed more light on the Application and limitations of molecular diagnostic tools. Product introductions are another factor in the market's growth. For instance, to increase India's molecular diagnostics capacity for COVID-19 and beyond, the Centre for Cellular and Molecular Platforms (C-CAMP) created InDx 2.0 National Diagnostics Catapult in February 2023. The Catapult, C-CAMP InDx 2.0, aims to improve India's preparedness for ongoing and upcoming pandemics and expand India's diagnostics capabilities for infectious diseases, including but not limited to COVID-19.
POC molecular diagnostics market aims to improve the diagnosis process and speed up patient treatment accurately. It will help reduce the patient's stay in healthcare facilities and help specialists treat more critical patients. In the past few decades, infectious diseases and chronic illnesses like cancer have increased. For instance, As per WHO, seasonal influenza results in 290,000-650,000 yearly mortalities globally. In 2020, WHO estimated 374 million new infections with 1 of 4 STIs: chlamydia (129 million), gonorrhea (82 million), syphilis (7.1 million), and trichomoniasis (156 million).
Rapid diagnosis and accurate treatment will help reduce the prevalence of such diseases. The market is segmented as follows.
Key Market Trends in Global Point-of-Care Molecular Diagnostics Industry
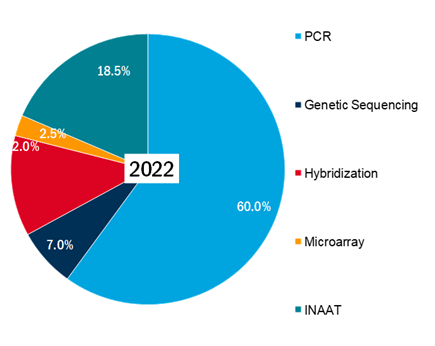
• According to OMR analysis, By Technology, sub-segment PCR held the largest market share and is projected to grow at a CAGR of 9.0% during the forecast period (2022-2030). The market share for technology sub-segments for the year 2022 is as follows-
According to OMR analysis, By Region, North America held a significant market share and is expected to grow at a CAGR of 8.3% over the forecast period (2022-2030).
The research report has an in-depth analysis of all the segments such as application, and end-user, and their market share in the global POC molecular diagnostics market for deeper insights and understanding.
The key market player in the industry is Abbott Laboratories, Becton, Dickinson, and Company, Siemens Healthineers Diagnostics, and F. Hoffmann-La Roche Ltd.’S. The report has included 20 other company profiles from the industry across the globe to understand the competitive landscape of the global POC molecular diagnostic industry.
Latest News in the Global POC Molecular Diagnostic Industry
• In March 2023, Illumina Inc., a genetic sequencing and array-based technology, announced the release of Connected Insights, a new cloud-based software enabling tertiary analysis for clinical next-generation sequencing (NGS) data.
• In February 2023, Pune-based Mylab Discovery Solutions launched three new rapid kits for the early and fast detection of sexually transmitted infections (STIs) such as Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), and Syphilis. The tests to detect sexually transmitted diseases are easy to use, capable of being stored at room temperature. They can be deployed at the point of care in resource-limited settings.
• In August 2022, Roche announced the launch of the Digital LightCycler? System, Roche’s first digital polymerase chain reaction (PCR) system. This next-generation system detects disease and is designed to accurately quantify trace amounts of specific DNA and RNA targets not typically detectable by conventional PCR methods.
• In May 2022, Meridian Bioscience, Inc., a provider of diagnostic testing solutions and life science raw materials, announced the continued expansion of its isothermal amplification product line with the launch of two new innovative master mixes, Lyo-Ready™ Direct DNA LAMP Saliva Mix and Lyo-Ready™ Direct RNA/DNA LAMP Saliva Mix.
Due to different market drivers, a lot is happening in the global point-of-care molecular diagnostics market in every segment. To get an in-depth understanding of the market, a custom market research report would help better. OMR has conducted diverse research in the market and created a professional report. You can also subscribe to the same for the best market trends and technology.